Why Are Chloroplasts Essential To The Survival Of Many Animals As Well As Plants?
All animals and most microorganisms rely on the continual uptake of big amounts of organic compounds from their environment. These compounds are used to provide both the carbon skeletons for biosynthesis and the metabolic energy that drives cellular processes. It is believed that the outset organisms on the primitive Globe had access to an affluence of the organic compounds produced by geochemical processes, just that about of these original compounds were used up billions of years ago. Since that fourth dimension, the vast majority of the organic materials required by living cells have been produced by photosynthetic organisms, including many types of photosynthetic bacteria.
The most avant-garde photosynthetic bacteria are the blue-green alga, which have minimal nutrient requirements. They use electrons from water and the energy of sunlight when they convert atmospheric CO2 into organic compounds—a process called carbon fixation. In the class of splitting water [in the overall reaction nH2O + nCO2

(CH2O) n + nO2], they likewise liberate into the atmosphere the oxygen required for oxidative phosphorylation. As we see in this department, it is thought that the development of cyanobacteria from more primitive photosynthetic leaner eventually made possible the evolution of abundant aerobic life forms.
In plants and algae, which developed much later on, photosynthesis occurs in a specialized intracellular organelle—the chloroplast. Chloroplasts perform photosynthesis during the daylight hours. The immediate products of photosynthesis, NADPH and ATP, are used by the photosynthetic cells to produce many organic molecules. In plants, the products include a low-molecular-weight sugar (usually sucrose) that is exported to run across the metabolic needs of the many nonphotosynthetic cells of the organism.
Biochemical and genetic evidence strongly advise that chloroplasts are descendants of oxygen-producing photosynthetic bacteria that were endocytosed and lived in symbiosis with primitive eucaryotic cells. Mitochondria are too generally believed to be descended from an endocytosed bacterium. The many differences betwixt chloroplasts and mitochondria are thought to reflect their different bacterial ancestors, too as their subsequent evolutionary deviation. Nevertheless, the fundamental mechanisms involved in light-driven ATP synthesis in chloroplasts are very like to those that we have already discussed for respiration-driven ATP synthesis in mitochondria.
The Chloroplast Is One Member of the Plastid Family of Organelles
Chloroplasts are the most prominent members of the plastid family of organelles. Plastids are present in all living found cells, each cell type having its own characteristic complement. All plastids share certain features. Virtually notably, all plastids in a particular plant species contain multiple copies of the same relatively pocket-size genome. In addition, each is enclosed past an envelope equanimous of 2 concentric membranes.
As discussed in Chapter 12 (see Figure 12-3), all plastids develop from proplastids, small organelles in the immature cells of plant meristems (Figure xiv-33A). Proplastids develop according to the requirements of each differentiated cell, and the type that is present is determined in large office by the nuclear genome. If a leaf is grown in darkness, its proplastids enlarge and develop into etioplasts, which have a semicrystalline array of internal membranes containing a yellow chlorophyll forerunner instead of chlorophyll. When exposed to light, the etioplasts rapidly develop into chloroplasts by converting this precursor to chlorophyll and by synthesizing new membrane pigments, photosynthetic enzymes, and components of the electron-send chain.

Effigy fourteen-33
Plastid diversity. (A) A proplastid from a root tip prison cell of a bean plant. Note the double membrane; the inner membrane has as well generated the relatively sparse internal membranes nowadays. (B) Three amyloplasts (a form of leucoplast), or starch-storing (more...)
Leucoplasts are plastids present in many epidermal and internal tissues that do not become green and photosynthetic. They are footling more than enlarged proplastids. A common class of leucoplast is the amyloplast (Figure 14-33B), which accumulates the polysaccharide starch in storage tissues—a source of sugar for futurity use. In some plants, such as potatoes, the amyloplasts can grow to be as big as an average animate being prison cell.
It is important to realize that plastids are not just sites for photosynthesis and the degradation of storage materials. Plants have too used their plastids to compartmentalize their intermediary metabolism. Purine and pyrimidine synthesis, most amino acrid synthesis, and all of the fatty acid synthesis of plants takes place in the plastids, whereas in animal cells these compounds are produced in the cytosol.
Chloroplasts Resemble Mitochondria Only Have an Extra Compartment
Chloroplasts carry out their free energy interconversions by chemiosmotic mechanisms in much the aforementioned mode that mitochondria do. Although much larger (Figure fourteen-34A), they are organized on the same principles. They have a highly permeable outer membrane; a much less permeable inner membrane, in which membrane send proteins are embedded; and a narrow intermembrane infinite in between. Together, these membranes form the chloroplast envelope (Figure xiv-34B,C). The inner membrane surrounds a big space chosen the stroma, which is analogous to the mitochondrial matrix and contains many metabolic enzymes. Like the mitochondrion, the chloroplast has its own genome and genetic organisation. The stroma therefore likewise contains a special ready of ribosomes, RNAs, and the chloroplast DNA.
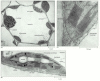
Effigy xiv-34
Electron micrographs of chloroplasts. (A) In a wheat leaf cell, a thin rim of cytoplasm—containing chloroplasts, the nucleus, and mitochondria—surrounds a large vacuole. (B) A thin department of a single chloroplast, showing the chloroplast (more...)
There is, even so, an important difference betwixt the organization of mitochondria and that of chloroplasts. The inner membrane of the chloroplast is not folded into cristae and does not incorporate electron-transport chains. Instead, the electron-transport chains, photosynthetic calorie-free-capturing systems, and ATP synthase are all contained in the thylakoid membrane, a 3rd distinct membrane that forms a ready of flattened disclike sacs, the thylakoids (Figure 14-35). The lumen of each thylakoid is idea to be connected with the lumen of other thylakoids, thereby defining a tertiary internal compartment called the thylakoid space, which is separated past the thylakoid membrane from the stroma that surrounds it.

Figure 14-35
The chloroplast. This photosynthetic organelle contains three distinct membranes (the outer membrane, the inner membrane, and the thylakoid membrane) that define three separate internal compartments (the intermembrane space, the stroma, and the thylakoid (more than...)
The structural similarities and differences between mitochondria and chloroplasts are illustrated in Figure 14-36. The head of the chloroplast ATP synthase, where ATP is made, protrudes from the thylakoid membrane into the stroma, whereas it protrudes into the matrix from the inner mitochondrial membrane.

Figure fourteen-36
A mitochondrion and chloroplast compared. A chloroplast is more often than not much larger than a mitochondrion and contains, in addition to an outer and inner membrane, a thylakoid membrane enclosing a thylakoid infinite. Unlike the chloroplast inner membrane, the (more...)
Chloroplasts Capture Energy from Sunlight and Use It to Fix Carbon
The many reactions that occur during photosynthesis in plants can be grouped into ii broad categories:
- 1.
-
In the photosynthetic electron-transfer reactions (also called the "light reactions"), energy derived from sunlight energizes an electron in the green organic pigment chlorophyll, enabling the electron to motion along an electron-transport chain in the thylakoid membrane in much the aforementioned way that an electron moves along the respiratory concatenation in mitochondria. The chlorophyll obtains its electrons from water (H2O), producing O2 every bit a by-product. During the electron-transport process, H+ is pumped across the thylakoid membrane, and the resulting electrochemical proton gradient drives the synthesis of ATP in the stroma. Every bit the concluding step in this series of reactions, high-energy electrons are loaded (together with H+) onto NADP+, converting information technology to NADPH. All of these reactions are confined to the chloroplast.
- 2.
-
In the carbon-fixation reactions (too chosen the "nighttime reactions"), the ATP and the NADPH produced past the photosynthetic electron-transfer reactions serve as the source of energy and reducing power, respectively, to drive the conversion of CO2 to carbohydrate. The carbon-fixation reactions, which begin in the chloroplast stroma and continue in the cytosol, produce sucrose and many other organic molecules in the leaves of the plant. The sucrose is exported to other tissues as a source of both organic molecules and energy for growth.
Thus, the germination of ATP, NADPH, and O2 (which requires light energy directly) and the conversion of COii to carbohydrate (which requires light free energy but indirectly) are separate processes (Figure 14-37), although elaborate feedback mechanisms interconnect the ii. Several of the chloroplast enzymes required for carbon fixation, for example, are inactivated in the nighttime and reactivated by calorie-free-stimulated electron-transport processes.
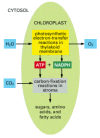
Figure 14-37
The reactions of photosynthesis in a chloroplast. H2o is oxidized and oxygen is released in the photosynthetic electron-transfer reactions, while carbon dioxide is assimilated (fixed) to produce sugars and a multifariousness of other organic molecules in the (more...)
Carbon Fixation Is Catalyzed past Ribulose Bisphosphate Carboxylase
Nosotros have seen before in this chapter how cells produce ATP by using the large amount of costless energy released when carbohydrates are oxidized to COii and HiiO. Conspicuously, therefore, the opposite reaction, in which COtwo and HtwoO combine to brand carbohydrate, must be a very unfavorable one that can only occur if it is coupled to other, very favorable reactions that drive it.
The central reaction of carbon fixation, in which an cantlet of inorganic carbon is converted to organic carbon, is illustrated in Effigy 14-38: CO2 from the atmosphere combines with the five-carbon compound ribulose 1,5-bisphosphate plus water to yield two molecules of the 3-carbon chemical compound three-phosphoglycerate. This "carbon-fixing" reaction, which was discovered in 1948, is catalyzed in the chloroplast stroma by a large enzyme called ribulose bisphosphate carboxylase. Since each molecule of the complex works sluggishly (processing only about three molecules of substrate per 2d compared to 1000 molecules per second for a typical enzyme), many enzyme molecules are needed. Ribulose bisphosphate carboxylase ofttimes constitutes more than 50% of the total chloroplast protein, and it is thought to be the nearly arable protein on Globe.
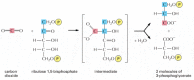
Figure 14-38
The initial reaction in carbon fixation. This reaction, in which carbon dioxide is converted into organic carbon, is catalyzed in the chloroplast stroma by the abundant enzyme ribulose bisphosphate carboxylase. The product is 3-phosphoglycerate, which (more...)
Three Molecules of ATP and Two Molecules of NADPH Are Consumed for Each CO2 Molecule That Is Fixed
The bodily reaction in which CO2 is fixed is energetically favorable because of the reactivity of the energy-rich chemical compound ribulose 1,5-bisphosphate, to which each molecule of CO2 is added (run across Figure fourteen-38). The elaborate metabolic pathway that produces ribulose 1,5-bisphosphate requires both NADPH and ATP; information technology was worked out in one of the first successful applications of radioisotopes every bit tracers in biochemistry. This carbon-fixation cycle (likewise called the Calvin cycle) is outlined in Figure 14-39. Information technology starts when 3 molecules of CO2 are stock-still by ribulose bisphosphate carboxylase to produce six molecules of three-phosphoglycerate (containing half dozen × three = 18 carbon atoms in all: 3 from the COtwo and fifteen from ribulose ane,5-bisphosphate). The 18 carbon atoms then undergo a cycle of reactions that regenerates the 3 molecules of ribulose 1,5-bisphosphate used in the initial carbon-fixation footstep (containing 3 × five = xv carbon atoms). This leaves ane molecule of glyceraldehyde three-phosphate (iii carbon atoms) every bit the net proceeds.
Figure 14-39
The carbon-fixation cycle, which forms organic molecules from CO2 and H2O. The number of carbon atoms in each type of molecule is indicated in the white box. There are many intermediates between glyceraldehyde 3-phosphate and ribulose 5-phosphate, but (more than...)
A total of 3 molecules of ATP and 2 molecules of NADPH are consumed for each CO2 molecule converted into carbohydrate. The cyberspace equation is:

Thus, both phosphate-bond free energy (every bit ATP) and reducing power (equally NADPH) are required for the germination of organic molecules from CO2 and HiiO. Nosotros return to this important point later.
The glyceraldehyde 3-phosphate produced in chloroplasts by the carbon-fixation cycle is a three-carbon sugar that likewise serves as a central intermediate in glycolysis. Much of it is exported to the cytosol, where it can exist converted into fructose six-phosphate and glucose 1-phosphate past the reversal of several reactions in glycolysis (see Console ii-8, pp. 124–125). The glucose 1-phosphate is then converted to the sugar nucleotide UDP-glucose, and this combines with the fructose vi-phosphate to class sucrose phosphate, the immediate precursor of the disaccharide sucrose. Sucrose is the major form in which carbohydrate is transported between institute cells: just equally glucose is transported in the blood of animals, sucrose is exported from the leaves via vascular bundles, providing the carbohydrate required past the rest of the institute.
Most of the glyceraldehyde 3-phosphate that remains in the chloroplast is converted to starch in the stroma. Like glycogen in animate being cells, starch is a big polymer of glucose that serves as a carbohydrate reserve (see Figure fourteen-33B). The production of starch is regulated and then that information technology is produced and stored as big grains in the chloroplast stroma during periods of excess photosynthetic capacity. This occurs through reactions in the stroma that are the reverse of those in glycolysis: they catechumen glyceraldehyde 3-phosphate to glucose 1-phosphate, which is and then used to produce the sugar nucleotide ADP-glucose, the firsthand precursor of starch. At night the starch is broken downwardly to help support the metabolic needs of the plant. Starch provides an of import part of the diet of all animals that consume plants.
Carbon Fixation in Some Plants Is Compartmentalized to Facilitate Growth at Low COtwo Concentrations
Although ribulose bisphosphate carboxylase preferentially adds CO2 to ribulose ane,5-bisphosphate, information technology can use O2 equally a substrate in place of CO2, and if the concentration of CO2 is low, it will add together O2 to ribulose 1,5-bisphosphate instead (see Effigy 14-38). This is the first step in a pathway chosen photorespiration, whose ultimate consequence is to use upwards O2 and liberate CO2 without the production of useful free energy stores. In many plants, near one-third of the CO2 fixed is lost again as CO2 considering of photorespiration.
Photorespiration can exist a serious liability for plants in hot, dry atmospheric condition, which cause them to shut their stomata (the gas exchange pores in their leaves) to avert excessive water loss. This in turn causes the CO2 levels in the leaf to fall precipitously, thereby favoring photorespiration. A special adaptation, however, occurs in the leaves of many plants, such as corn and saccharide cane that live in hot, dry environments. In these plants, the carbon-fixation bike occurs but in the chloroplasts of specialized parcel-sheath cells, which contain all of the plant's ribulose bisphosphate carboxylase. These cells are protected from the air and are surrounded by a specialized layer of mesophyll cells that use the energy harvested by their chloroplasts to "pump" COtwo into the packet-sheath cells. This supplies the ribulose bisphosphate carboxylase with a high concentration of COii, thereby greatly reducing photorespiration.
The COii pump is produced by a reaction cycle that begins in the cytosol of the mesophyll cells. A CO2-fixation pace is catalyzed by an enzyme that binds carbon dioxide (as bicarbonate) and combines it with an activated three-carbon molecule to produce a four-carbon molecule. The four-carbon molecule diffuses into the bundle-sheath cells, where it is broken down to release the CO2 and generate a molecule with three carbons. The pumping cycle is completed when this iii-carbon molecule is returned to the mesophyll cells and converted back to its original activated course. Because the COii is initially captured by converting it into a compound containing four carbons, the CO2-pumping plants are chosen C4 plants. All other plants are called C3 plants because they capture CO2 into the three-carbon compound 3-phosphoglycerate (Figure 14-40).

Effigy 14-40
Comparative leafage beefcake in a C3 institute and a C4 plant. The cells with dark-green cytosol in the leaf interior contain chloroplasts that perform the normal carbon-fixation cycle. In C4 plants, the mesophyll cells are specialized for COtwo pumping rather than (more than...)
Every bit for any vectorial send process, pumping CO2 into the bundle-sheath cells in C4 plants costs energy. In hot, dry environments, however, this cost can be much less than the energy lost by photorespiration in C3 plants, and so C4 plants accept a potential advantage. Moreover, because Cfour plants can perform photosynthesis at a lower concentration of CO2 inside the leaf, they need to open their stomata less often and therefore can prepare near twice as much net carbon as C3 plants per unit of water lost. Although the vast majority of plant species are Cthree plants, C4 plants such as corn and sugar pikestaff are much more effective at converting sunlight free energy into biomass than C3 plants such as cereal grains. They are therefore of special importance in world agriculture.
Photosynthesis Depends on the Photochemistry of Chlorophyll Molecules
Having discussed the carbon-fixation reactions, nosotros at present return to the question of how the photosynthetic electron-transfer reactions in the chloroplast generate the ATP and the NADPH needed to bulldoze the product of carbohydrates from COtwo and H2O. The required energy is derived from sunlight absorbed past chlorophyll molecules (Figure 14-41). The process of energy conversion begins when a chlorophyll molecule is excited past a quantum of light (a photon) and an electron is moved from one molecular orbital to some other of college energy. As illustrated in Figure 14-42, such an excited molecule is unstable and tends to render to its original, unexcited state in 1 of three ways:
Figure 14-41
The structure of chlorophyll. A magnesium cantlet is held in a porphyrin band, which is related to the porphyrin band that binds iron in heme (run across Figure fourteen-22). Electrons are delocalized over the bonds shown in blue.

Figure 14-42
Iii means for an excited chlorophyll molecule to render to its original, unexcited land. The low-cal free energy absorbed by an isolated chlorophyll molecule is completely released as light and oestrus by process ane. In photosynthesis, past contrast, chlorophylls (more...)
- 1.
-
By converting the extra energy into heat (molecular motions) or to some combination of heat and light of a longer wavelength (fluorescence), which is what happens when light energy is absorbed by an isolated chlorophyll molecule in solution.
- 2.
-
By transferring the energy—but non the electron—directly to a neighboring chlorophyll molecule by a process chosen resonance energy transfer.
- 3.
-
By transferring the high-free energy electron to another nearby molecule, an electron acceptor, and so returning to its original state by taking up a low-free energy electron from some other molecule, an electron donor.
The last ii mechanisms are exploited in the process of photosynthesis.
A Photosystem Consists of a Reaction Center Plus an Antenna Complex
Multiprotein complexes called photosystems catalyze the conversion of the light energy captured in excited chlorophyll molecules to useful forms. A photosystem consists of 2 closely linked components: an antenna complex, consisting of a large set up of pigment molecules that capture light energy and feed it to the reaction middle; and a photochemical reaction center, consisting of a complex of proteins and chlorophyll molecules that enable light energy to exist converted into chemical free energy (Figure 14-43).
Figure 14-43
The antenna complex and photochemical reaction center in a photosystem. The antenna complex is a collector of light energy in the form of excited electrons. The energy of the excited electrons is funneled, through a series of resonance energy transfers, (more...)
The antenna complex is important for capturing lite. In chloroplasts it consists of a number of singled-out membrane protein complexes (known equally light-harvesting complexes); together, these proteins bind several hundred chlorophyll molecules per reaction center, orienting them precisely in the thylakoid membrane. Depending on the plant, different amounts of accessory pigments chosen carotenoids, which protect the chlorophylls from oxidation and can help collect light of other wavelengths, are also located in each complex. When a chlorophyll molecule in the antenna complex is excited, the energy is rapidly transferred from one molecule to another by resonance free energy transfer until information technology reaches a special pair of chlorophyll molecules in the photochemical reaction center. Each antenna complex thereby acts as a funnel, collecting calorie-free energy and directing it to a specific site where information technology can be used finer (run into Figure 14-43).
The photochemical reaction center is a transmembrane protein-paint complex that lies at the heart of photosynthesis. It is thought to have evolved more than 3 billion years ago in primitive photosynthetic bacteria. The special pair of chlorophyll molecules in the reaction center acts as an irreversible trap for excitation quanta considering its excited electron is immediately passed to a chain of electron acceptors that are precisely positioned as neighbors in the same protein circuitous (Figure xiv-44). By moving the high-free energy electron chop-chop away from the chlorophylls, the photochemical reaction centre transfers it to an surroundings where it is much more than stable. The electron is thereby suitably positioned for subsequent reactions, which require more time to consummate.

Effigy xiv-44
The arrangement of the electron carriers in a bacterial photochemical reaction middle, as determined by x-ray crystallography. The paint molecules shown are held in the interior of a transmembrane protein and are surrounded by the lipid bilayer of the (more...)
In a Reaction Center, Low-cal Energy Captured past Chlorophyll Creates a Strong Electron Donor from a Weak One
The electron transfers involved in the photochemical reactions just outlined have been analyzed extensively past rapid spectroscopic methods. An enormous amount of detailed data is available for the photosystem of purple bacteria, which is somewhat simpler than the evolutionarily related photosystems in chloroplasts. The reaction heart in this photosystem is a large protein-pigment complex that can exist solubilized with detergent and purified in active grade. In 1985, its complete 3-dimensional structure was adamant past x-ray crystallography (see Figure 10-38). This construction, combined with kinetic information, provides the best picture nosotros have of the initial electron-transfer reactions that underlie photosynthesis.
The sequence of electron transfers that take place in the reaction center of purple bacteria is shown in Effigy fourteen-45. As outlined previously for the full general case (see Effigy 14-43), calorie-free causes a net electron transfer from a weak electron donor (a molecule with a stiff affinity for electrons) to a molecule that is a strong electron donor in its reduced course. The excitation energy in chlorophyll that would normally be released every bit fluorescence or heat is thereby used instead to create a strong electron donor (a molecule carrying a high-energy electron) where none had been earlier. In the regal bacterium, the weak electron donor used to fill the electron-deficient hole created past a calorie-free-induced accuse separation is a cytochrome (come across orange box in Figure xiv-45); the strong electron donor produced is a quinone. In the chloroplasts of college plants, a quinone is similarly produced. However, as we hash out side by side, water serves as the initial weak electron donor, which is why oxygen gas is released past photosynthesis in plants.

Figure 14-45
The electron transfers that occur in the photochemical reaction center of a purple bacterium. A similar set of reactions occurs in the evolutionarily related photosystem Ii in plants. At the top left is an orientating diagram showing the molecules that (more...)
Noncyclic Photophosphorylation Produces Both NADPH and ATP
Photosynthesis in plants and cyanobacteria produces both ATP and NADPH directly by a two-stride process called noncyclic photophosphorylation. Because two photosystems—called photosystems I and Two—are used in series to energize an electron, the electron can be transferred all the way from water to NADPH. Every bit the loftier-energy electrons laissez passer through the coupled photosystems to generate NADPH, some of their energy is siphoned off for ATP synthesis.
The first of the ii photosystems—paradoxically called photosystem II for historical reasons—has the unique ability to withdraw electrons from water. The oxygens of two water molecules demark to a cluster of manganese atoms in a poorly understood water-splitting enzyme. This enzyme enables electrons to exist removed one at a time from the water, as required to fill the electron-scarce holes created past light in chlorophyll molecules in the reaction heart. Every bit soon every bit iv electrons take been removed from the two h2o molecules (requiring 4 quanta of low-cal), Oii is released. Photosystem II thus catalyzes the reaction 2HtwoO + 4 photons → 4H+ + 4e - + Otwo. As nosotros discussed for the electron-transport chain in mitochondria, which uses Otwo and produces water, the mechanism ensures that no partly oxidized water molecules are released as unsafe, highly reactive oxygen radicals. Essentially all the oxygen in the Earth'southward temper has been produced in this way.
The core of the reaction centre in photosystem II is homologous to the bacterial reaction center just described, and it likewise produces strong electron donors in the class of reduced quinone molecules dissolved in the lipid bilayer of the membrane. The quinones pass their electrons to a H+ pump called the cytochrome b6-f circuitous, which resembles the cytochrome b-c one complex in the respiratory concatenation of mitochondria. The cytochrome b 6 -f complex pumps H+ into the thylakoid space across the thylakoid membrane (or out of the cytosol across the plasma membrane in blue-green alga), and the resulting electrochemical gradient drives the synthesis of ATP by an ATP synthase (Figure 14-46).

Effigy 14-46
Electron menses during photosynthesis in the thylakoid membrane. The mobile electron carriers in the chain are plastoquinone (which closely resembles the ubiquinone of mitochondria), plastocyanin (a small copper-containing protein), and ferredoxin (a small (more...)
The last electron acceptor in this electron-transport concatenation is the second photosystem, photosystem I, which accepts an electron into the electron-deficient hole created by light in the chlorophyll molecule in its reaction center. Each electron that enters photosystem I is finally additional to a very loftier-energy level that allows it to be passed to the iron-sulfur eye in ferredoxin and then to NADP+ to generate NADPH (Effigy 14-47).

Figure 14-47
Changes in redox potential during photosynthesis. The redox potential for each molecule is indicated by its position along the vertical centrality. In photosystem Two, the excited reaction center chlorophyll has a redox potential high enough to withdraw electrons (more than...)
The scheme for photosynthesis just discussed is known as the Z scheme. By means of its two electron-energizing steps, ane catalyzed past each photosystem, an electron is passed from h2o, which normally holds on to its electrons very tightly (redox potential = +820 mV), to NADPH, which normally holds on to its electrons loosely (redox potential = -320 mV). There is non enough energy in a unmarried breakthrough of visible light to energize an electron all the way from the bottom of photosystem II to the summit of photosystem I, which is presumably the energy change required to pass an electron efficiently from h2o to NADP+. The apply of two separate photosystems in serial means that the free energy from ii quanta of light is available for this purpose. In addition, at that place is enough energy left over to enable the electron-transport concatenation that links the ii photosystems to pump H+ across the thylakoid membrane (or the plasma membrane of cyanobacteria), so that the ATP synthase can harness some of the lite-derived energy for ATP product.
Chloroplasts Tin can Brand ATP by Circadian Photophosphorylation Without Making NADPH
In the noncyclic photophosphorylation scheme just discussed, high-free energy electrons leaving photosystem II are harnessed to generate ATP and are passed on to photosystem I to drive the production of NADPH. This produces slightly more than 1 molecule of ATP for every pair of electrons that passes from H2O to NADP+ to generate a molecule of NADPH. Simply 1.5 molecules of ATP per NADPH are needed for carbon fixation (see Figure 14-39). To produce extra ATP, the chloroplasts in some species of plants can switch photosystem I into a cyclic mode and so that it produces ATP instead of NADPH. In this process, called circadian photophosphorylation, the high-energy electrons from photosystem I are transferred to the cytochrome b half-dozen -f complex rather than being passed on to NADP+. From the b 6 -f complex, the electrons are passed back to photosystem I at a depression free energy. The only net result, besides the conversion of some light energy to heat, is that H+ is pumped across the thylakoid membrane past the b 6 -f circuitous every bit electrons pass through information technology, thereby increasing the electrochemical proton slope that drives the ATP synthase. (This is analogous to the correct side of the diagram for purple nonsulfur bacteria in Figure fourteen-71, beneath.)
To summarize, cyclic photophosphorylation involves only photosystem I, and it produces ATP without the germination of either NADPH or O2. The relative activities of cyclic and noncyclic electron flows tin can be regulated past the cell to make up one's mind how much low-cal energy is converted into reducing power (NADPH) and how much into high-energy phosphate bonds (ATP).
Photosystems I and II Have Related Structures, and Also Resemble Bacterial Photosystems
The mechanisms of primal prison cell processes such as DNA replication or respiration more often than not plow out to be the same in eucaryotic cells and in bacteria, even though the number of protein components involved is considerably greater in eucaryotes. Eucaryotes evolved from procaryotes, and the additional proteins presumably were selected for during evolution because they provided an extra degree of efficiency and/or regulation that was useful to the prison cell.
Photosystems provide a clear case of this type of evolution. Photosystem Two, for example, is formed from more than 25 dissimilar protein subunits, creating a big assembly in the thylakoid membrane with a mass of virtually 1 one thousand thousand daltons. The diminutive structures of the eucaryotic photosystems are being revealed by a combination of electron and ten-ray crystallography. The task is difficult because the complexes are large and embedded in the lipid bilayer. Nevertheless, as illustrated in Effigy 14-48, the shut human relationship of photosystem I, photosystem II, and the photochemical reaction eye of royal bacteria has been clearly demonstrated from these atomic-level analyses.

Figure 14-48
Three types of photosynthetic reaction centers compared. Pigments involved in lite harvesting are colored green; those involved in the central photochemical events are colored crimson. (A) The photochemical reaction center of royal bacteria, whose detailed (more...)
The Proton-Motive Strength Is the Same in Mitochondria and Chloroplasts
The presence of the thylakoid space separates a chloroplast into three rather than the ii internal compartments of a mitochondrion. The net effect of H+ translocation in the two organelles is, however, similar. As illustrated in Figure fourteen-49, in chloroplasts, H+ is pumped out of the stroma (pH 8) into the thylakoid space (pH ~five), creating a gradient of 3–3.5 pH units. This represents a proton-motive force of nigh 200 mV beyond the thylakoid membrane, and it drives ATP synthesis past the ATP synthase embedded in this membrane. The forcefulness is the same as that beyond the inner mitochondrial membrane, but almost all of it is contributed past the pH slope rather than by a membrane potential, different the instance in mitochondria.

Figure fourteen-49
A comparison of the menstruum of H+ and the orientation of the ATP synthase in mitochondria and chloroplasts. Those compartments with similar pH values have been colored the same. The proton-motive strength beyond the thylakoid membrane consists nearly entirely (more...)
Like the stroma, the mitochondrial matrix has a pH of near eight. This is created by pumping H+ out of the mitochondrion into the cytosol (pH ~7) rather than into an interior space in the organelle. Thus, the pH gradient is relatively modest, and most of the proton-motive force across the inner mitochondrial membrane is instead acquired by the resulting membrane potential (see Effigy 14-13).
For both mitochondria and chloroplasts, the catalytic site of the ATP synthase is at a pH of about 8 and is located in a large organelle compartment (matrix or stroma) that is packed full of soluble enzymes. Consequently, it is here that all of the organelle'south ATP is fabricated (see Figure fourteen-49).
Carrier Proteins in the Chloroplast Inner Membrane Control Metabolite Commutation with the Cytosol
If chloroplasts are isolated in a fashion that leaves their inner membrane intact, this membrane tin can exist shown to have a selective permeability, reflecting the presence of specific carrier proteins. Most notably, much of the glyceraldehyde 3-phosphate produced by COii fixation in the chloroplast stroma is transported out of the chloroplast by an efficient antiport system that exchanges three-carbon carbohydrate phosphates for an inward flux of inorganic phosphate.
Glyceraldehyde 3-phosphate unremarkably provides the cytosol with an abundant source of carbohydrate, which is used by the jail cell every bit the starting point for many other biosyntheses—including the product of sucrose for consign. But this is not all that this molecule provides. Once the glyceraldehyde iii-phosphate reaches the cytosol, it is readily converted (by part of the glycolytic pathway) to 1,iii-phosphoglycerate and and then 3-phosphoglycerate (run into p. 97), generating ane molecule of ATP and one of NADH. (A similar ii-step reaction, but working in reverse, forms glyceraldehyde three-phosphate in the carbon-fixation cycle; come across Effigy fourteen-39.) Equally a result, the consign of glyceraldehyde three-phosphate from the chloroplast provides not just the primary source of fixed carbon to the rest of the prison cell, only also the reducing power and ATP needed for metabolism outside the chloroplast.
Chloroplasts Also Perform Other Crucial Biosyntheses
The chloroplast performs many biosyntheses in add-on to photosynthesis. All of the cell's fatty acids and a number of amino acids, for example, are made by enzymes in the chloroplast stroma. Similarly, the reducing power of light-activated electrons drives the reduction of nitrite (NO2 -) to ammonia (NHiii) in the chloroplast; this ammonia provides the plant with nitrogen for the synthesis of amino acids and nucleotides. The metabolic importance of the chloroplast for plants and algae therefore extends far beyond its role in photosynthesis.
Summary
Chloroplasts and photosynthetic bacteria obtain loftier-energy electrons by ways of photosystems that capture the electrons that are excited when sunlight is absorbed by chlorophyll molecules. Photosystems are composed of an antenna circuitous that funnels energy to a photochemical reaction middle, where a precisely ordered complex of proteins and pigments allows the free energy of an excited electron in chlorophyll to be captured by electron carriers. The best-understood photochemical reaction center is that of royal photosynthetic leaner, which contain merely a single photosystem. In contrast, at that place are ii distinct photosystems in chloroplasts and blue-green alga. The ii photosystems are unremarkably linked in series, and they transfer electrons from water to NADP+ to form NADPH, with the concomitant production of a transmembrane electrochemical proton gradient. In these linked photosystems, molecular oxygen (O2) is generated as a by-production of removing 4 low-free energy electrons from 2 specifically positioned water molecules.
Compared with mitochondria, chloroplasts have an boosted internal membrane (the thylakoid membrane) and a third internal space (the thylakoid space). All electron-transport processes occur in the thylakoid membrane: to make ATP, H+ is pumped into the thylakoid space, and a backflow of H+ through an ATP synthase then produces the ATP in the chloroplast stroma. This ATP is used in conjunction with the NADPH fabricated by photosynthesis to drive a large number of biosynthetic reactions in the chloroplast stroma, including the all-important carbon-fixation cycle, which creates carbohydrate from CO2. Along with some other important chloroplast products, this carbohydrate is exported to the jail cell cytosol, where—every bit glyceraldehyde 3-phosphate—information technology provides organic carbon, ATP, and reducing power to the rest of the cell.
Source: https://www.ncbi.nlm.nih.gov/books/NBK26819/
Posted by: childressinks1998.blogspot.com

0 Response to "Why Are Chloroplasts Essential To The Survival Of Many Animals As Well As Plants?"
Post a Comment